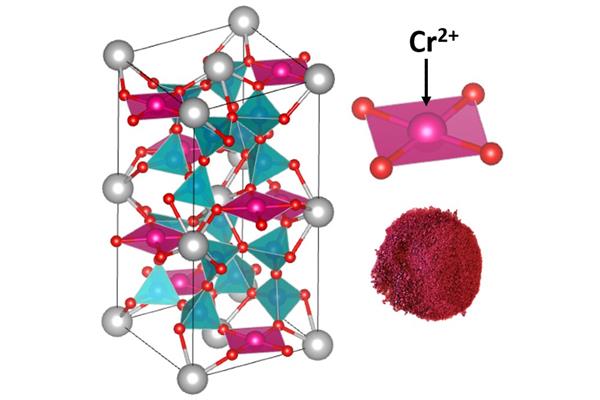
The researchers from the Oregon State University have developed new chromium-based reddish magenta pigments taking inspiration from the moon.
A group of researchers from the Oregon State University has recently published a new study
illustrating how it has developed durable chromium-based reddish magenta pigments inspired by lunar mineralogy and ancient Egyptian chemistry, which can be employed during the manufacturing process of energy-efficient coatings for vehicles and buildings.
The new pigments are based on divalent chromium (Cr2+) and are the first to use it as a chromophore – the part of a molecule that determine colour by reflecting some wavelengths of light while absorbing others. The study was inspired by the divalent copper that serves as a chromophore in Egyptian blue, which is the first known synthetic pigment and dates to more than 5,000 years ago.
The researchers replaced the divalent copper in Egyptian blue with divalent chromium, leading to durable reddish magenta pigments. In order to stabilise the divalent chromium on Earth, researchers maintained high temperatures under high vacuum during the synthesis that started from chromium metal, chromium trioxide and other chemicals.
“Most of the magenta-coloured pigments used today are organic chemicals and suffer from stability issues when exposed to ultraviolet rays and heat from the sun because they can break down organic chemical. Inorganic magenta pigments are rare, and most require a significant number of cobalt salts that are hazardous to both humans and the environment,” has stated Mas Subramanian, the professor of materials science from the Oregon State University who led the study. “To date, no earth-based mineral has been reported to contain chromium in the divalent state as one of the components. However, the analysis of lunar mineral samples collected from Apollo missions showed the occurrence of chromium in the divalent state.”
The magenta pigments developed by the researchers are thermally and chemically inert because of their high preparation temperature and remain unaltered structurally and optically upon exposure to acid and alkali. In addition, unlike pigments that contain cobalt, the chromium-based magenta pigments are highly reflective of heat from the sun – meaning they have a cooling property that would lead to energy savings for cars and structures coated with them.
“Most pigments are discovered by chance. The reason is because the origin of the colour of a material depends not only on the chemical composition but also on the intricate arrangement of atoms in the crystal structure. So, someone has to make the material first in a laboratory, then study its crystal structure thoroughly to explain the colour. Despite recent advances in quantum mechanical theories and computational methods, predicting a crystal structure that will produce an intense inorganic pigment of a desired colour is still elusive”, has added Subramanian.
New colours might be discovered
Divalent chromium has the same number of unpaired electrons as trivalent manganese, the chromophore responsible for the intense colour of YInMn blue, discovered by the same team fifteen years ago. The researchers had been experimenting with new materials that could be used in electronics applications and mixed manganese oxide – which is black in colour – with other chemicals, then heated them in a furnace. One of their samples turned out to be a brilliant blue, named YInMn blue after the component elements yttrium, indium and manganese. It was the first blue pigment discovery in two centuries and a huge advance in safety and durability, as well as vividness.
“We got lucky the first time with YInMn blue, and now we are coming up with some fundamental chemical and crystal structural design principles to rationally create new pigments. Determining the key structural ingredients required for making vivid colours should allow for shorter times between pigment discoveries. Science does not always follow a prescribed path, but we are exploring pigments with divalent chromium as a chromophore in diverse coordination environments in crystal structures of various inorganic compounds,” has concluded Subramanian.