One of the most popular methods to ensure corrosion protection of difficult-to-reach assets, such as underground or submerged pipelines, is cathodic protection. Developed in the first half of the 19th century to protect the metal hulls of warships, it is now widely used not only on large structures but also on the inside of water heaters or boilers for private use. Let us take a detailed look at its main features and applications.
What is cathodic protection?
Cathodic protection is an electrochemical corrosion protection system that makes the metal surfaces to be protected more negative than its redox potential in contact with electrolytes. In practice, direct current is circulated between an electrode (anode) placed in the environment (electrolyte) and the surface of the structure to be protected (cathode): by lowering the potential of the metallic material to be protected, such current reduces the speed of corrosion to zero or at least to a negligible value. Current circulation can be activated by galvanic coupling with a less noble metal or through a current generator whose positive pole is connected to a suitable earth electrode whereas the negative pole is connected to the structure to be protected.
Let us now go into more detail, starting with a few basic principles.
Corrosion
is a natural surface process of material degradation governed by chemical and physical laws. Metal corrosion can be classified according to the environment in which it occurs: wet (metal in contact with an environment containing water) or dry (metal in contact with gaseous atmospheres at high temperatures). In the first case, corrosion, specifically electrochemical corrosion, triggers two reactions: an anodic reaction of metal dissolution (release of electrons) and a cathodic reaction of reduction (consumption of the electrons produced).
When a metal is in contact with an electrolyte
(water, soil, moisture, etc.), it takes on an electrical potential determined by these chemical reactions, the value of which varies depending on the type of metal and electrolyte. When two different metals are in electrical contact with each other and immersed in an electrolyte, they assume two different potential values, which creates a flow of spontaneous electric current. This tends to lead their natural potentials towards an identical value called corrosion or mixed potential. The circulation of current in the electrolyte occurs by ionic migration linked to the cathodic reactions of reduction and oxidation (redox) and thus to the disintegration of the metal whose potential is more anodic.
When applying external electric current on the metals pair, however, it is possible to change the mixed potential value, resulting in overvoltage. If overvoltage is such that the mixed potential decreases to a value below the potential of the most anodic metal, the corrosion reaction can no longer take place. This is the concept behind the cathodic protection
technique, which is now widely used to protect underground and submerged metal structures and concrete reinforcements against corrosion. The first experiments in this field are attributed to Sir Humphry Davy: in the first half of the 19th
century, his discoveries initiated the replacement of wooden-hulled warships with metal-hulled ones properly protected against the aggression of the highly saline environment of the sea. On 22 January 1824, at the Royal Society’s premises, Davy explained that, when the electrical state of a metallic material becomes more negative, it is possible to completely eliminate the forces that are generated and to prevent corrosion from occurring. He had the idea of using iron, tin, and zinc anodes to make copper more negative and thus eliminate the problem of corrosion. His results laid the foundations of the cathodic protection technology, making it possible to use metallic materials in maritime structures in complete safety.
 © KorrosionGruppen AB
© KorrosionGruppen ABCathodic protection: how to apply it
Cathodic protection, therefore, consists in making the metal surface to be protected more electronegative than its redox potential in contact with a given electrolyte. Of course, current must be supplied through a circuit capable of circulating it in the electrolyte with an ion exchange fed by the oxidation reaction. Therefore, using a DC generator is not enough, but it is also necessary to include an expendable element (anode) on which the oxidation reaction can take place.
There are two methods of applying cathodic protection systems: the galvanic system and the impressed current system. The choice of one or other type depends on the size of the surface area to be protected, the condition of the metal structure’s coating, and the characteristics of the electrolyte (seawater for maritime structures or soil for land-based ones), always also taking the economic aspect into account.
The galvanic system
The galvanic system uses galvanic or sacrificial anodes made of special magnesium, zinc, or aluminium alloys. The potential difference between the metal (usually steel) and the anode causes an electric current to flow through the electrolyte. Current circulation changes the potential of the metal with respect to the electrolyte, preventing the formation of corrosion cells, i.e. those particular cells that convert chemical energy into electrical energy thus causing the corrosion phenomenon.
In general, the main advantages of galvanic cathodic protection are as follows:
- there is no risk of interference with other metal structures;
- there is no risk of overprotection;
- the supply of electricity is not necessary;
- in most cases, installation costs are lower;
- maintenance is reduced to a minimum.
On the other hand, its main disadvantages are follows:
- the current generated is limited by the potential difference between the anode and the metal structure;
- there is a risk of reduced efficiency, if the electrical resistivities of water or soil are not low enough (maximum 6,000 ohms.cm);
- protection may not be optimal if no coating has been applied or if the coating is not effective, and with large-sized metal structures.
The impressed current system
This system uses an external current source (cathodic protection rectifiers) and special inert anodes. The most commonly used anodes are ferro-silicon-chromium and titanium. In this case, the protective electric current is generated by the rectifier and not by the potential difference between the structure and the anode.
The main advantages of this system are follows:
- it provides larger amounts of protective current and allows controlling it;
- it can be applied to any type of water or soil, even those with high electrical resistivity;
- it can be effectively applied for protecting bare or uncoated metal structures.
However, impressed current systems have the following disadvantages:
- they need regular maintenance operations, although easy to implement;
- they need electricity, although minimal.
The most common applications of cathodic protection systems
Originally developed in the first half of the 19th century to prevent corrosion of metal hulls, cathodic protection has since expanded its fields of application. In fact, it is now particularly used to protect large metal structures such as underground or submerged structures (e.g. pipelines or the bottoms of storage tanks in industrial plants), metal reinforcements of concrete buildings or bridges, or internal parts of widely used items such as water heaters and boilers.
Let us take a look at the most common uses in detail, taking as reference some articles already published on the subject.
Underground pipes and storage tank bottoms
In industrial plants, it is very likely that cracks will appear in the bottoms of tanks, in the pipes of the fire-fighting system, and in the cooling water network after a few years of operation. In order to avoid this, it is important to study the behaviour of soil as a corrosive agent.
Soil corrosion depends on numerous variables, such as aeration, humidity, pH, the presence of microorganisms, climatic conditions, inhomogeneity, and the presence of sulphate-reducing bacteria, fertilisers, industrial waste, and chemicals of various kinds. Such complex situation can be further aggravated by any defects in the lining of the underground pipes and tank bottoms and, above all, by the steel/copper galvanic coupling phenomenon caused by the presence of the industrial plant’s electrical earthing circuit, which is essential for electrical safety but extremely harmful in terms of corrosion. Finally, under even more adverse conditions, the pipes or tanks of some industrial plants may also be affected by leakage currents from electrified railway networks, nearby cathodic protection systems, operating welding equipment, and other sources of direct current. Such leakage currents damage underground metal structures through forced electrolytic corrosion, causing some of them to crack before the plant has even begun to operate. These numerous variables mean that soil is considered one of the most complex existing corrosive agents, since it is impossible to determine precisely how aggressive it will be towards the metallic materials (usually carbon steel) buried in it.
On the other hand, corrosion issues in the underground structures of an industrial plant can be diagnosed with a good degree of accuracy by identifying and analysing the following variables:
- electrical resistivity of the soil;
- pH value of the soil;
- pipelines and tanks’ potentials measured in relation to the soil itself;
- installation characteristics of tanks, underground pipes, and electrical earthing systems (structure, length, diameter, type of coating, and installation plans).
Once the presence of corrosion has been diagnosed in underground pipelines and storage tanks, the installation of a cathodic protection system can prevent corrosion initiation regardless of its type (soil, galvanic, leakage current-related, or all of these together). Impressed current cathodic protection systems, in particular, are widely used in industrial plants: one or more rectifiers and inert iron/silicon/chromium anodes are distributed within the plant and buried up to 3 metres deep.
 © Amparo Engineering Limited
© Amparo Engineering LimitedUnderwater assets
In recent years, both types of cathodic protection systems have been widely used for the protection of large submarine pipelines, mining platforms, Floating Production Storage and Offloading (FPSO) units, ships, boats, and port structures built with steel piles and tubular piles, but also in the construction of metal roofs and pylons in general.
Submarine pipelines
Extensive underwater pipelines, such as those connecting offshore fields to onshore structures, are very well coated and they use galvanic systems that are easy to install and do not require any power supply. Anodes are often in the form of clamps or bracelets and they are supplied with the pipeline for a protection period of more than thirty years. Smaller or near-shore pipelines, such as submarine outfalls or pipes connecting to nearby platforms, are often protected with already existing rectifiers located on the coast or on platforms.
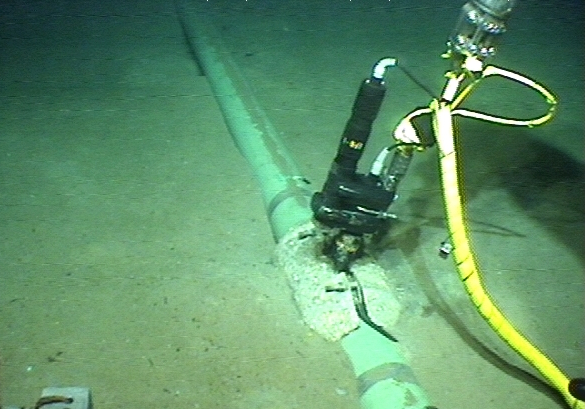 © Deepwater
© DeepwaterFPSOs
Galvanic or impressed current systems can be used for Floating Production Storage and Offloading (FPSO) units. It is important to emphasise that, in these cases, galvanic systems offer more advantages, as they do not present any risk of overprotection and electrical interference in the pillars’ pipes, as well as being able to be installed immediately at the start of construction.
Mooring pylons and metal covers
Metal pylons and covers located close to the coast and intended for mooring ships and boats are built with steel piles or tubular piles that feature large surfaces to be protected, which are generally not coated or painted. Impressed current systems are widely used in these structures because of the large amounts of current required.
Hulls of large ships
The hulls of large ships used for transporting oil, minerals, seeds, and other goods are protected with impressed current systems, with the help of automatic rectifiers and reference electrodes, which make it possible to control protection potentials and the efficiency of the system. Galvanic anodes are widely used for the internal protection of these vessels’ tanks.
Restoration of cathodic protection on old submerged structures
Both galvanic and impressed current systems are widely used for restoring the proper cathodic protection levels in old metal structures where the original system is worn and inefficient. In this case, electrical cables are connected to the pipelines and metal structures quickly, easily, and safely without the need for underwater welding, with the help of special patented devices that enable to achieve and maintain excellent electrical contact even in submerged pipelines with high coating thicknesses.
Metal reinforcements in concrete
Under normal conditions, concrete has an alkaline pH of approximately 12.5. In this ideal environment, reinforcements
enter a passive state with virtually zero corrosion. However, this condition does not always prevail: in the presence of salts (usually chlorides) or when the concrete coating is carbonated, the reinforcements’ steel becomes susceptible to corrosion. The products resulting from the corrosion of steel occupy two to ten times the volume occupied by the original metallic material, generating an enormous pressure (over 15 MPa) in the space between concrete and steel. The progressive cracking and scaling of concrete facilitates the penetration of aggressive agents and the diffusion of O2 and CO2, accelerating the corrosion process and culminating in the collapse of the structure.
We are all accustomed to observing the occurrence of this phenomenon in the most diverse types of concrete structures. In these cases, corrosion is an electrochemical phenomenon caused by the circulation of electrical currents from corrosion stacks, which are commonly found in carbon steel. It is necessary to prevent such corrosion stacks, i.e. sets of galvanic cells placed in series, from functioning. In practice, this can be achieved very easily and cheaply by installing galvanic cathodic protection anodes.
These anodes, which can have different shapes and which completely solve the corrosion problem, should be installed during the construction of new structures (as a preventive measure) or during the repair of existing structures that have already been corroded. They are alkali-activated and supplied as small components in a special zinc alloy, encapsulated with a suitable material that makes them active when embedded in cement. They feature looped cables that can be easily connected to the metal parts of the concrete structure. Installation is simple and easy and it can be done in less than a minute.
 © Vector Corrosion Technologies
© Vector Corrosion TechnologiesWater heaters and boilers
Continuous contact of metal with less noble materials immersed in water within water heaters and boilers leads to the initiation of corrosion on uncoated areas. The aggressiveness of water increases proportionally as the temperature rises (particularly above 60 °C). This is why sacrificial anodes, usually made of magnesium, are introduced to generate a small current flow that adequately protects the structure. In order for this protection to be effective, the type and the correct positioning of anodes are key to achieve the right current supply, necessary to protect the tank in all its parts and reach the protection potential near any defects as quickly as possible.
 © AdobeStock
© AdobeStockConclusions
Cathodic protection is referred to as an indispensable tool against corrosion by the laws of many countries around the world, particularly when it comes to protecting submerged pipelines. Indeed, it can guarantee a high safety and efficiency degree, essential in any industrial plant: the damage that could be caused by the triggering of corrosion in such large and strategic sites for energy supply is invaluable not only in terms of costs but also of environmental protection. In other words, these structures must be safeguarded against corrosion by all means – and cathodic protection is one of them.
 © Wikipedia
© Wikipedia